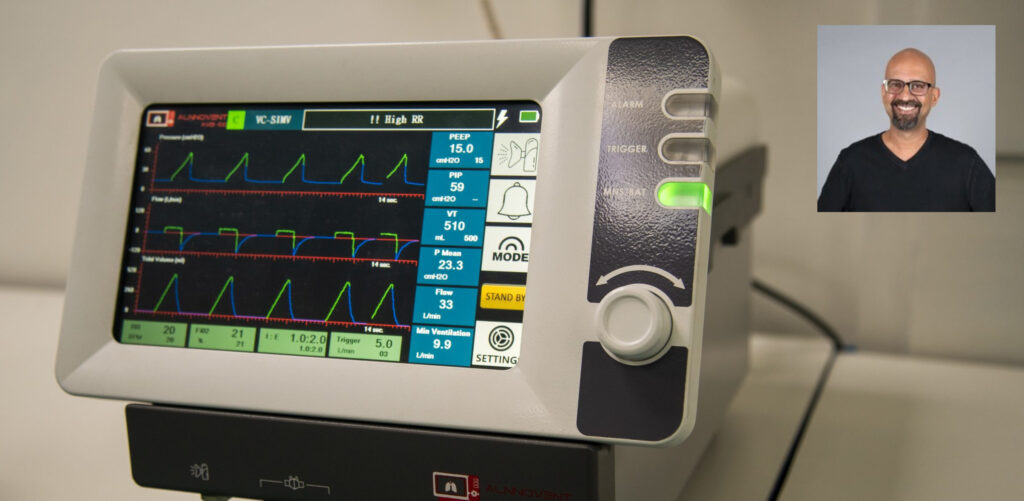
‘Innovation is the unrelenting drive to break the status quo and develop anew where few have dared to go.’ – Steven Jeffes This is what Alsons Technology achieved in 2024. The AlnnoVent AVB–100, a state-of-the-art Intensive Care Unit (ICU) ventilator conceived, designed, and manufactured at Alsons’ industrial setup in Pakistan, broke the status quo—challenging the longstanding reluctance to develop and manufacture medical devices in Pakistan, which had led to an over[1]reliance on imported biomedical devices.
This achievement has undoubtedly opened the doors for the local manufacturing of not only electro medical devices, but should also inspire others to replicate this success in other engineering sectors within Pakistan. Like every extraordinary effort, the AVB–100 has a long history that will surely pave the way for future proponents of Pakistan-made engineering miracles. The most vibrant aspect of this history is that it was entirely homegrown, born out of a sheer disappointment at the shortage of ventilators in our ICUs during the onslaught of the Covid-19 pandemic, whilst thousands of our fellow citizens were fighting for their lives.
Alsons took on the challenge to create an ICU ventilator that could not only meet the emergency needs of the pandemic but also help make Pakistan self-sufficient in the production of electro medical devices—a feat many deemed impossible due to the country’s limited engineering expertise and regulatory challenges.
Amidst the countrywide lockdowns, making this device seemed almost impossible. People were confined to their homes, the availability of raw materials was a challenge, and our engineers faced numerous risks in the wake of the Covid-19 pandemic’s deadly waves.
It was a great commitment to the people of Pakistan and a deep desire to contribute to the “Made in Pakistan” effort that led us to develop the AVB-100— the very first Pakistan[1]made ventilator, which was ready for engineering validations by the Pakistan Engineering Council (PEC).
The PEC and its Pakistan Innovation and Testing Centre (PITC), then led by the highly competent Engr. Brig. Tariq Javed and his team of brilliant young engineers, gave us the initial engineering guidelines and standards, performed engineering tests and, after due diligence approved the AVB- 100 for the next, and perhaps most significant and difficult, phase of approval—the human clinical trials.
Now, a new and unknown chapter was about to unfold—the role of the Drug Regulatory Authority of Pakistan (DRAP). Another key player in this process was the National Bioethics Committee (NBC). These two significant organizations ensured that the biomedical devices about to be connected with human lives were 100% safe for patients.
This was the biggest challenge. The Terms of Reference set for the clinical trials were not only comprehensive but also laid the foundation for future biomedical devices, especially ventilators, in Pakistan. Alsons along with DRAP, learned valuable lessons during this phase, and the clinical trials proceeded as planned. Over 1,000 kilometers away from its birthplace in Karachi, the AVB-100 was tested in the ICUs of Jinnah Hospital, Lahore.
Alsons ensured that its team of biomedical engineers was present at the trial site to collect data, which was concurrently analyzed by the Data Safety and Management Board (DSMB). The study, based on the baseline data, provided valuable insights for future studies. Prof. Dr. Shabbir Jumani led the DSMB, which included Prof. Dr. Noor-ul-Haq, Dr. Hamid Mehmood, Dr. Engr. Abdul Aleem Jamali, and Prof. Dr. Fouzia Sadiq.
The Human Clinical Trials at Jinnah Hospital were initially led by Dr. Hina Nabi and concluded by Prof Ashraf Zia and his team. The trials were conducted over a period of two years providing us an opportunity to fully test and qualify our device under the stringent regulatory framework provided by DRAP.
The clinical trial reports and studies of the AVB[1]100 were presented before the Clinical Studies Committee and the Medical Device Board at DRAP, where it finally received approval for manufacturing in December of last year.
In addition to all the years of effort and commitment, another chapter of collective achievement was being written in Pakistan. A large number of healthcare professionals, including Prof. Dr. Shahid Noor, the vanguard of Saman-e-Shifa Foundation, and his incredible team, Prof. Dr. Tipu Sultan and Pakistan Society of Anesthesiologists, played an instrumental role in helping and training the Alsons team on various aspects of the ventilator’s application and clinical use.
The unwavering support of Government institutions such as Ministry of Science & Technology, Ministry of National Health Services Regulations and Coordination, PEC/PITC, DRAP, NBC and the Special Investment Facilitation Council (SIFC) has now resulted in the reality of today—the birth of locally[1]made biomedical devices in Pakistan. – The author is Director at Alsons Group
Appeared in Samane-Shifa Newsletter