Electrospray ionization mass spectrometry or, less frequently, electrospray mass spectrometry are the two names for ESI-based mass spectrometry.
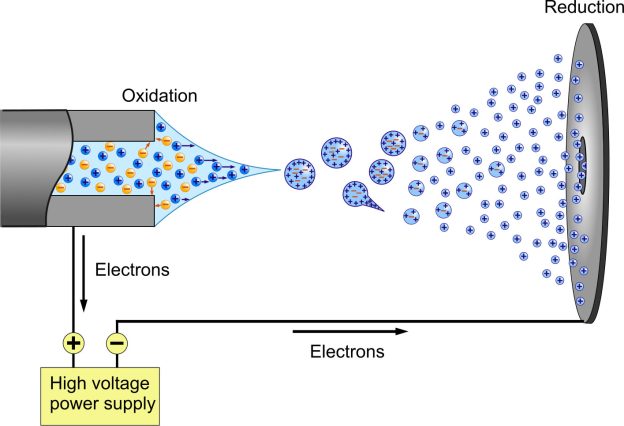
ESI is referred to as a “soft ionization” approach because there is hardly any fragmentation. Although the molecular ion (or more precisely, a pseudo molecular ion) is almost always detected, this can be helpful because very little structural information can be gleaned from the straightforward mass spectrum. Electrospray is used to disperse the liquid containing the target analytes into a fine aerosol. The usual solvents for electrospray ionization are made by combining water with volatile organic molecules since the ion production requires considerable solvent evaporation (e.g. methanol acetonitrile). Compounds that improve conductivity, such as acetic acid, are typically added to the solution to reduce the initial droplet size. Additionally, these species serve as a source of protons to speed up the ionization process. In addition to the high temperature of the ESI source, large-flow electrosprays can benefit from the nebulization of a heated inert gas like nitrogen or carbon dioxide.
The ions detected by mass spectrometry may be quasimolecular ions, which are denoted [M + H]+ when a hydrogen cation is added, [M + Na]+ when a sodium ion is added, or [M H] when a hydrogen nucleus is removed. Multiple-charged ions, such [M + nH]n+, are frequently seen. Numerous charge states can exist in massive macromolecules, creating a distinctive charge state envelope.
Because of the significantly smaller initial droplets created by the electrosprays when they are operated at low flow rates, ionization efficiency is increased. Significant sensitivity gains could be achieved with lower flow rates, as low as 200 nL/min, according to a 1993 study by Gale and Richard D. Smith. Two research teams came up with the term micro-electrospray (microspray) in 1994 to describe electrosprays that operate at low flow rates. Emmett and Caprioli showed that operating the electrospray at 300–800 nL/min resulted in increased performance for HPLC–MS analyses. Wilm and Mann showed that an electrospray at the tip of emitters made by drawing glass capillaries to a few micrometres may be sustained at a capillary flow of less than 25 nL/min.
In a two-step procedure called laser-based electrospray-based ambient ionization, material from a sample is desorbed or ablated using a pulsed laser, and then a plume of that material interacts with an electrospray to produce ions. The sample substance is deposited on a target close to the electrospray for ambient ionization. Material from the sample is ejected from the surface and into the electrospray, which creates highly charged ions, when the laser desorbs or ablates it. These include laser ablation electrospray ionization, matrix-assisted laser desorption electrospray ionization, and electrospray laser desorption ionization. The study of noncovalent gas phase interactions also makes use of electrospray ionization. It is believed that noncovalent compounds from the liquid phase can be transferred into the gas phase using the electrospray method without affecting the non-covalent interaction. When analyzing ligand substrate complexes by ESI-MS or nanoESI-MS, issues such non-specific interactions have been found. Investigating the relationships between enzymes and medications that function as their inhibitors is a fascinating example of this. ESI has been employed in competition experiments between STAT6 and inhibitors to screen for potential novel medication candidates. Smaller droplets are produced and only a few microliters of a sample are consumed during nano-electrospray ionization. The reduced electrospray droplet size made it possible to perform successful desolvation and ion production at low flow rates, which was a specific advantage of operating at low pressure.
Variants of Electrospray Ionization
on 20/11/2023